Radiography Testing

Radiography Testing uses X-rays or gamma-rays to produce an image of an object on film. The image is usually natural-size. X-rays and gamma-rays are very short wavelength electromagnetic radiation which can pass through solid material, being partly absorbed during transmission. Thus, if an X-ray source is placed on one side of a specimen and a photographic film on the other side, an image is obtained on the film of the thickness variations in the specimen, whether these are surface or internal.
Advantage
The flaws can be detected in the following Areas,
- Weldments, Castings, Forgings & tubes
- Pipelines, Storage tanks & Pressure vessels
- Electronic, Electrical & Mechanical Components
- Structural Steel
X-Radiation
X-rays are just like any other kind of electromagnetic radiation. They can be produced in parcels of energy called photons, just like light. There are two different atomic processes that can produce X-ray photons. One is called Bremsstrahlung and is a German term meaning “braking radiation.” The other is called K-shell emission. They can both occur in the heavy atoms of tungsten. Tungsten is often the material chosen for the target or anode of the x-ray tube.
Both ways of making X-rays involve a change in the state of electrons. However, Bremsstrahlung is easier to understand using the classical idea that radiation is emitted when the velocity of the electron shot at the tungsten changes. The negatively charged electron slows down after swinging around the nucleus of a positively charged tungsten atom. This energy loss produces X-radiation. Electrons are scattered elastically and inelastically by the positively charged nucleus. The inelastically scattered electron loses energy, which appears as Bremsstrahlung. Elastically scattered electrons (which include backscattered electrons) are generally scattered through larger angles.
In the interaction, many photons of different wavelengths are produced, but none of the photons have more energy than the electron had to begin with. After emitting the spectrum of X-ray radiation, the original electron is slowed down or stopped.
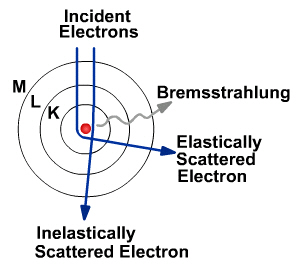
Bremsstrahlung Radiation
X-ray tubes produce x-ray photons by accelerating a stream of electrons to energies of several hundred kilovolts with velocities of several hundred kilometers per hour and colliding them into a heavy target material. The abrupt acceleration of the charged particles (electrons) produces Bremsstrahlung photons. X-ray radiation with a continuous spectrum of energies is produced with a range from a few keV to a maximum of the energy of the electron beam. Target materials for industrial tubes are typically tungsten, which means that the wave functions of the bound tungsten electrons are required. The inherent filtration of an X-ray tube must be computed, which is controlled by the amount that the electron penetrates into the surface of the target and by the type of vacuum window present.
The bremsstrahlung photons generated within the target material are attenuated as they pass through typically 50 microns of target material. The beam is further attenuated by the aluminum or beryllium vacuum window. The results are an elimination of the low energy photons, 1 keV through l5 keV, and a significant reduction in the portion of the spectrum from 15 keV through 50 keV. The spectrum from an x-ray tube is further modified by the filtration caused by the selection of filters used in the setup.
The applet below allows the user to visualize an electron accelerating and interacting with a heavy target material. The graph keeps a record of the bremsstrahlung photons numbers as a function of energy. After a few events, the “building up” of the graph may be accomplished by pressing the “automate” button.
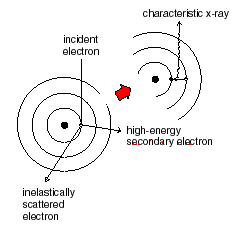
K-shell Emission Radiation
Remember that atoms have their electrons arranged in closed “shells” of different energies. The K-shell is the lowest energy state of an atom. An incoming electron can give a K-shell electron enough energy to knock it out of its energy state. About 0.1% of the electrons produce K-shell vacancies; most produce heat. Then, a tungsten electron of higher energy (from an outer shell) can fall into the K-shell. The energy lost by the falling electron shows up in an emitted x-ray photon. Meanwhile, higher energy electrons fall into the vacated energy state in the outer shell, and so on. K-shell emission produces higher-intensity x-rays than Bremsstrahlung, and the x-ray photon comes out at a single wavelength.
When outer-shell electrons drop into inner shells, they emit a quantized photon “characteristic” of the element. The energies of the characteristic X-rays produced are only very weakly dependent on the chemical structure in which the atom is bound, indicating that the non-bonding shells of atoms are the X-ray source. The resulting characteristic spectrum is superimposed on the continuum as shown in the graphs below. An atom remains ionized for a very short time (about 10-14 second) and thus an atom can be repeatedly ionized by the incident electrons which arrive about every 10-12 second.
Gamma Radiation
Gamma radiation is one of the three types of natural radioactivity. Gamma rays are electromagnetic radiation, like X-rays. The other two types of natural radioactivity are alpha and beta radiation, which are in the form of particles. Gamma rays are the most energetic form of electromagnetic radiation, with a very short wavelength of less than one-tenth of a nanometer.
Gamma radiation is the product of radioactive atoms. Depending upon the ratio of neutrons to protons within its nucleus, an isotope of a particular element may be stable or unstable. When the binding energy is not strong enough to hold the nucleus of an atom together, the atom is said to be unstable. Atoms with unstable nuclei are constantly changing as a result of the imbalance of energy within the nucleus. Over time, the nuclei of unstable isotopes spontaneously disintegrate, or transform, in a process known as radioactive decay. Various types of penetrating radiation may be emitted from the nucleus and/or its surrounding electrons. Nuclides which undergo radioactive decay are called radionuclides. Any material which contains measurable amounts of one or more radionuclides is a radioactive material.


Types Radiation Produced by Radioactive Decay
When an atom undergoes radioactive decay, it emits one or more forms of radiation with sufficient energy to ionize the atoms with which it interacts. Ionizing radiation can consist of high speed subatomic particles ejected from the nucleus or electromagnetic radiation (gamma-rays) emitted by either the nucleus or orbital electrons.
Alpha Particles
Certain radionuclides of high atomic mass (Ra226, U238, Pu239) decay by the emission of alpha particles. These alpha particles are tightly bound units of two neutrons and two protons each (He4 nucleus) and have a positive charge. Emission of an alpha particle from the nucleus results in a decrease of two units of atomic number (Z) and four units of mass number (A). Alpha particles are emitted with discrete energies characteristic of the particular transformation from which they originate. All alpha particles from a particular radionuclide transformation will have identical energies.
Beta Particles
A nucleus with an unstable ratio of neutrons to protons may decay through the emission of a high speed electron called a beta particle. This results in a net change of one unit of atomic number (Z). Beta particles have a negative charge and the beta particles emitted by a specific radionuclide will range in energy from near zero up to a maximum value, which is characteristic of the particular transformation.
Gamma-rays
A nucleus which is in an excited state may emit one or more photons (packets of electromagnetic radiation) of discrete energies. The emission of gamma rays does not alter the number of protons or neutrons in the nucleus but instead has the effect of moving the nucleus from a higher to a lower energy state (unstable to stable). Gamma ray emission frequently follows beta decay, alpha decay, and other nuclear decay processes.
